As early as 1989, A.Y. of the University of Berkeley in the United States Liu and M.L. Cohen theoretically predicted a material synthesised from carbon and nitrogen, inferred that its hardness could be comparable to that of diamond, or even higher than diamond, and found that it had many excellent characteristics such as high heat resistance, but the theory and the manufacture of these materials are two different things. So far, there have not been many successful studies on synthesis. Scientists have been trying to synthesise this compound in the laboratory by various means, but the results are not ideal.
Until recently, Dominique Laniel and colleagues at the University of Edinburgh in the United Kingdom compressed the carbon and nitrogen between diamond drill bits at a pressure of 700,000 times atmospheric pressure and heated it to 3,000 degrees Celsius with a laser, finally synthesising this compound almost as hard as diamond - a carbon nitride. Tiny samples. Researchers published this result in the recently published Advanced Materials.
This newly synthesised carbon nitride is almost as hard as diamond. The hardness of diamond is about 90GPa, while the hardness of the previously known second hard material, cubic boron nitride, is between 50GPa and 55GPa. Laniel said that the hardness of the new carbon nitride material they synthesised is between 78GPa and 86GPa, depending on which of the three crystalline structures formed.
The research results show that the three synthetic carbon nitride compounds have the structure required by breakthrough super-hard materials. Scientists are surprised to find that these three compounds still retain their super-hard properties after cooling and returning to environmental pressure.
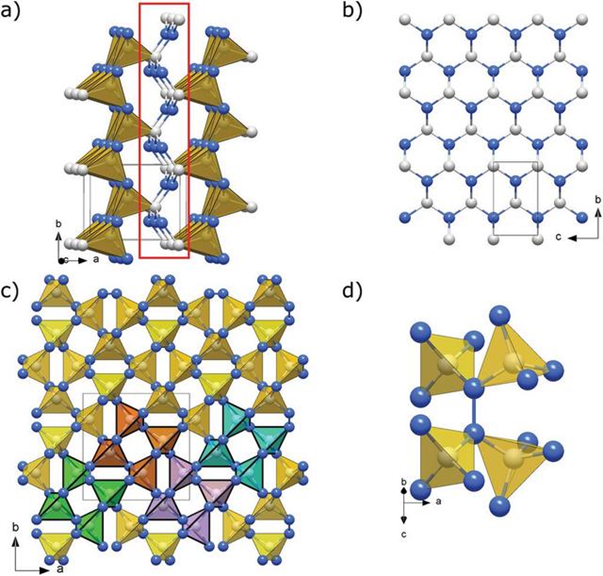
However, the hardness of carbon nitride synthesised by Laniel and others overturned the 1989 prediction that the hardness of the substance would exceed diamond.
There is a huge gap in hardness between cubic boron nitride and diamond, which ranked second in hardness before. And our synthesis results make up for this gap. Laniel said that although their newly synthesised material is called "carbon nitride", they are two very different elements. They are very willing to accept broader opinions and give them a more accurate name.
At present, the synthesised samples are only 5 microns wide and 3 microns thick, so it may be difficult to expand the production scale. However, in theory, using larger diamonds to compress carbon and nitrogen should produce larger blocks of materials. If the compound is successfully developed, it is expected to be used in the manufacture of cutting tools, sensors and even explosives. But the pressure required for synthesis may be higher, which will make the cost of carbon nitride much higher than that of diamond.
Laniel said that this new material has advantages that diamond does not have, such as generating electrical signals under pressure, which may make it applicable to the field of sensors. In addition, the material has a high energy density and may be made into a powerful explosive, but the environmental toxicity is much less. The team also believes that this breakthrough paves the way for multiple uses, including protective coatings for vehicles and spacecraft, powerful cutting tools and photoelectric detectors.
Source: China Science News, IT Home
